Infrared Spectroscopy
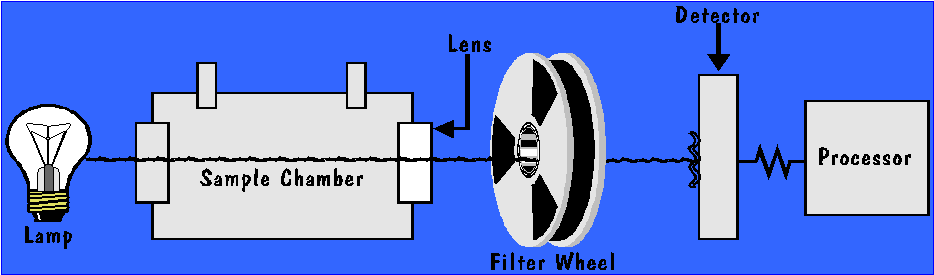
INFRARED ANALYZER
The above diagram provides a graphic representation of the basic design of an infrared analyzer which is used to measure breath alcohol concentrations. The design is based on the fact that specific wavelengths of infrared energy are absorbed by ethyl alcohol molecules. In its simplest form, the instrument’s detector measures the change in the amount of a specific wavelength of infrared energy that passes from the infrared source (lamp), through the sample chamber and filter wheel to the detector. The change in response on the detector, as a breath sample is submitted to the sample chamber , is monitored and analyzed by a processor in the instrument. The change in the signal is used to calculate an alcohol concentration
The difference between the amount of infrared energy that reaches the detector when the sample chamber is free of compounds that absorb the infrared energy and the amount of infrared energy that reaches the detector when a subject’s breath sample is within the sample chamber, provides an indication of the concentration of the absorbing substances in the sample . If ethanol was the only molecule found in a breath sample that would absorb energy at the wavelength being recorded by the detector, the calculated difference in infrared energy reaching the detector could be used by itself to establish the concentration of alcohol in the breath sample. Unfortunately this is not always the case.
In order to deal with the lack of specificity, it is important that the primary wavelength of infrared energy used to measure the concentration of alcohol is selected based upon its limited cross sensitivity to other substances that are commonly found in the human breath. The spinning filter wheel in the diagram above is used to modulate the light through several different filters allowing different wavelengths of infrared energy to be transmitted to the detector for analysis. The secondary wavelengths of energy are selected based upon the interfering compounds that could be found in a human breath sample. If these compounds are identified and are in concentrations that would adversely effect the calculated ethanol result, the analysis can be aborted.
One other important point is that the differing concentrations of alcohol or other energy absorbing compounds found in the subject’s breath sample are not in a one to one relationship with the amount of energy that reaches the detector. In other words, a .050 alcohol concentration may absorb X units of infrared energy, but a .200 will not absorb 4X units of infrared energy. This inherent non-linearity can be overcome by performing a multi-point calibration. To ensure that the instrument is properly quantifying alcohol and the other substances, it would be prudent to perform multi-point accuracy checks for each substance of interest and ensure proper calibration over the entire range of substances and concentrations.
Finally, infrared systems require power to light the IR source, heat the sample chamber, power the light modulator and drive the detector and associated circuits. Historically, the signal from the detector has been small relative to the noise generated in the system. This has made it difficult to resolve changes in the signal when low level concentrations of alcohol are presented to the system. To solve this problem, several manufacturers have included cooled detectors. These systems require additional power, but the cooled detector decreases the noise on the detector and enhances the instrument’s performance at the low alcohol concentrations.
| Strengths of Infrared Based Analyzers | Weaknesses of Infrared Based Analyzers |
| Analyzes the breath sample continuously. Although it is commonly believed that the instrument can analyze a sample in real time, the dynamics of the sample inlet and sample chamber retard the detector’s ability to truly generate real time data. Nonetheless, there is valuable information in the continuous stream of data that is generated. | Cross reactivity in the ethanol absorption band to substances found on the breath other than alcohol – Requires multiple wavelengths to identify these substances – Requires Calibration Checking of interfering substances to ensure that these interfering substance systems are working properly. |
| This technology is commonly used for generating evidential breath alcohol results. | Noise in the system limits low level accuracy. |
| Infrared testing systems can perform analyses in rapid succession. Once the sample chamber is purged from the last sample and a new “zero” baseline established, a subject can provide another sample for analysis.
|
Since the detector output and concentration of alcohol are not a one to one relationship. Multiple point accuracy checks should be performed to ensure that the system is calculating alcohol concentrations and/or potential interfering substances properly throughout the full range of analysis. |
| Single chamber systems have difficulty establishing a true .000 baseline. | |
| Limited life of the infrared source. | |
| Breath chambers tend to be large and limit the ability of the system to capture consistent deep lung samples or determine subtle changes in alcohol concentrations in the breath sample. | |
| Infrared systems tend to be relatively large and require a large amount of power to operate. | |
| The current systems are costly relative to other available technologies. |
