About Alcohol
![]()
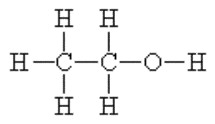
Alcohol is a Drug
When an alcoholic beverage is consumed it passes down the esophagus through the stomach and into the small intestine. Although a small amount of alcohol is absorbed into the bloodstream through the mucous membrane, the vast majority of alcohol enters the bloodstream through the walls of the small intestine. Alcohol is water soluble and the bloodstream rapidly transports the ethanol throughout the body where it is absorbed into the body tissues in proportion to their water content.
Ethanol is greatly diluted by the body fluids. For example, a 1-ounce shot of 80-proof whiskey, which contains 0.4 fluid ounces of ethanol will be diluted in a 150-pound human, producing somewhere in the neighborhood of an 0.02% blood alcohol concentration. With a user that is smaller with say one half of the water weight in his or her body than the individual in the prior example, that same 0.4 fluid ounce of ethanol would likely produce an alcohol concentration at or near 0.04%.
How Alcohol is Eliminated from the Body
Metabolism is the body’s process of converting ingested substances to other compounds. Metabolism involves a number of processes, one of which is referred to as oxidation. Through oxidation in the liver, alcohol is detoxified and removed from the blood, preventing the alcohol from accumulating and destroying cells and organs. A minute amount of alcohol escapes metabolism and is excreted unchanged in the breath, in the sweat and in urine. Until all the alcohol consumed has been metabolized, it is distributed throughout the body, affecting the brain and other tissues.
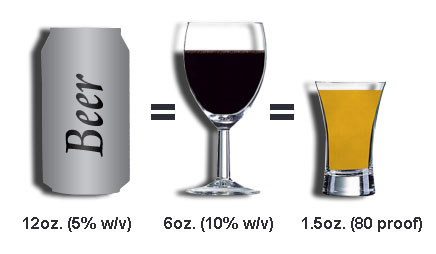
The liver can metabolize only a certain amount of alcohol per hour, regardless of the amount that has been consumed. The rate of alcohol metabolism depends, in part, on the amount of metabolizing enzymes in the liver, which varies among individuals. In general, after the consumption of one standard drink, the amount of alcohol in the drinker’s blood reaches maximum concentration within 30 to 90 minutes from the time that drinking stops. (A standard drink is defined as 12 ounces of beer, 6 ounces of wine, or 1.5 ounces of 80-proof distilled spirits, all of which contain the same amount of alcohol.) Alcohol is metabolized more slowly than it is absorbed. Since the metabolism of alcohol is slow, consumption needs to be controlled to prevent accumulation in the body and intoxication.
Factors Influencing Alcohol Absorption and Metabolism
Food. A number of factors influence the absorption process, including the presence of food and the type of food in the gastrointestinal tract when alcohol is consumed. The rate at which alcohol is absorbed depends on how quickly the stomach empties its contents into the intestine. The higher the dietary fat content, the more time this emptying will require and the longer the process of absorption will take. One study found that subjects who drank alcohol after a meal that included fat, protein, and carbohydrates absorbed the alcohol about three times more slowly than when they consumed alcohol on an empty stomach. Normally, total absorption occurs within 120 – 150 minutes after the cessation of alcohol consumption.
Gender. Generally, women and men metabolize alcohol differently. As a result, they will likely have higher Blood Alcohol Concentration’s (BAC) after consuming the same amount of alcohol as a similar sized man. The difference in BAC’s between women and men has been attributed to women’s smaller amount of body water, likened to dropping the same amount of alcohol into a smaller pail of water. An additional factor contributing to the difference in BAC’s may be that women have lower activity of the alcohol metabolizing enzyme ADH in the stomach, causing a larger proportion of the ingested alcohol to reach the blood. The combination of these factors may render women more vulnerable than men to alcohol-induced liver and heart damage as a result of heavy drinking.
If the amount of ethanol consumed is not great, the oxidization of the alcohol can keep up with the rate that the ethanol is entering the blood stream and the alcohol concentration will not increase,. (The ethanol disposal rate in a 150-pound human is about 0.5 ounce of ethanol per hour, which corresponds to12 ounces of beer, 6 ounces of wine, or 1.5 ounces of hard liquor.) If however, the alcohol intake is greater than the rate at which the user is able to metabolize it, the blood and breath alcohol concentration of that individual will increase.
How Alcohol Gets From the Blood into the Breath
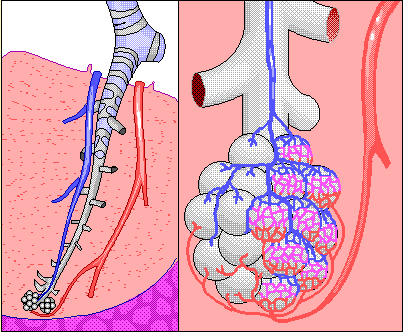 Ethanol is volatile and as a result, an amount of alcohol, in proportion to the concentration in the blood, transfers from the blood into the alveolar air sacs in the lungs. This occurs in much the same way that carbon dioxide leaves the alveolar blood and enters the lungs for exhalation from the body. As a result, it is possible to analyze an alveolar breath sample, determine the breath alcohol concentration (BrAC) and predict with a high degree of accuracy, the blood alcohol concentration at that same point in time.
Ethanol is volatile and as a result, an amount of alcohol, in proportion to the concentration in the blood, transfers from the blood into the alveolar air sacs in the lungs. This occurs in much the same way that carbon dioxide leaves the alveolar blood and enters the lungs for exhalation from the body. As a result, it is possible to analyze an alveolar breath sample, determine the breath alcohol concentration (BrAC) and predict with a high degree of accuracy, the blood alcohol concentration at that same point in time.
Alcohol is a Drug
Ethanol acts as a drug affecting the central nervous system. Its behavioral effects are a result of its influence on the response in the nervous tissue. It suppresses certain brain functions. Alcohol is a depressant, and high concentrations of ethanol can be a mild general anesthetic. It suppresses certain brain functions. At very low doses, it can appear to be a stimulant by suppressing certain inhibitory brain functions. However, as concentration increases, further suppression of nervous tissue functions produce the classic symptoms of intoxication: slurred speech, unsteady gate, disturbed sensory perceptions, and inability to react quickly. At high concentrations, ethanol produces general anesthesia; a highly intoxicated person will be in a coma like state and very difficult to wake. In extreme cases, if the alcohol concentration is high enough, it will inhibit basic involuntary bodily functions such as breathing and can cause death.

A Brief History of Breath Alcohol Testing
Alcohol levels in the brain are difficult to measure. As a result, blood alcohol levels were first used to assess the concentration of alcohol in a person’s brain tissue. It was determined that most people begin to show the first signs of mental impairment at around 0.05% blood alcohol. At around 0.10% mental impairment begins to become more obvious; motor impairment is measurable. The first signs of slurred speech show up at around 0.15%. When a user reaches 0.18% muscular incoordination is normally obvious and the user shows signs of disorientation. Vomiting and unconsciousness are consistent with users that achieve a 0.4% blood alcohol concentration. Above 0.5%, the breathing center of the brain or the beating action of the heart can be anesthetized, resulting in death.
Although there are advantages when testing with blood to determine alcohol concentrations in the human body, the sample collection process can be viewed as both invasive, painful and the analysis process time consuming and costly. In the 1930’s technology was created that took advantage of the fact that alcohol was found in the deep lung breath in proportion the alcohol found in the blood. Breath testing instruments were manufactured to capture a sample for analysis. Breath analysis has since evolved into a technology that offers a low cost, highly accurate, rapid analysis of a breath sample that is simply and painlessly collected.
Technologies that have been used to test a breath sample for alcohol include the following:
- Wet Chemistry
- Photo Spectroscopy
- Gas Chromatography
- Infrared Spectroscopy
- Tin Oxide Sensors
- Electrochemical Analysis
Learn more about Intoximeters’ role in the development of Breath Alcohol Testing by clicking About Us.
To learn more about the Alcohol and the Human Body, click Physiology and Pharmacology of Alcohol.
To learn more about fuel cell technology used for breath alcohol detection click Fuel Cell White Paper.
To view Breath Alcohol Testing Instruments by Intoximeters, click Breath Testing Instruments.
